Life Science Enhancement - Chemistry 4 - Carbon the element of life
Content
Scheme of work
C4.1 - Carbon’s Bonding Rules and Molecular Geometry
In grade 9, you learned about atomic structure and how electrons are arranged in shells. In this lesson, we focus on carbon — an element with unique bonding properties that allow it to form an enormous range of compounds. Carbon is the foundation of organic chemistry because it is tetravalent: it can form four covalent bonds.
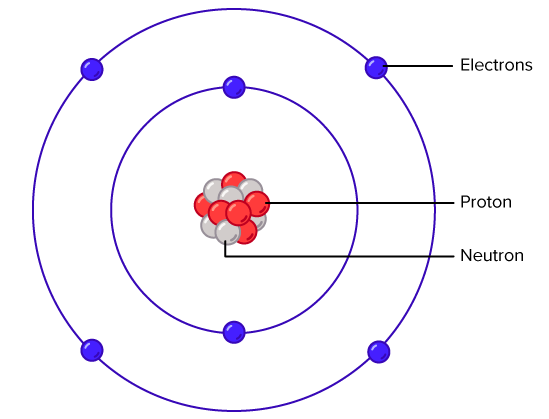
Carbon atom
Why is carbon tetravalent?
Carbon has four valence electrons in its outer shell. To achieve stability, it shares these electrons with others, forming four covalent bonds. These bonds arrange themselves to minimise repulsion, which gives rise to predictable molecular shapes.
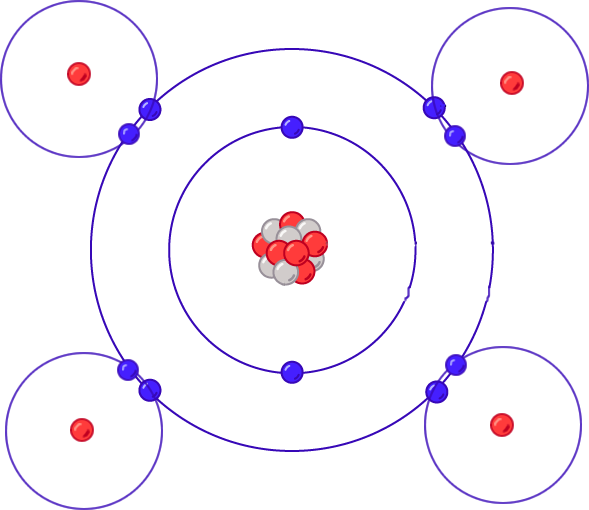
Methane molecule
Activity 1: Map Pin Molecular Geometry
Map Pin Challenge
The aim of the following activity is to explain why carbon bonds in a tetrahedral arrangement when bonding to four other atoms.
In groups, use large polystyrene spheres and map pins:
- Place 2, 3, 4 and 5 pins as far apart as possible on the surface.
- Identify the shapes adopted (linear, trigonal planar, tetrahedral, trigonal bipyramidal).
- Estimate bond angles using geometry and trigonometry.
Extension: use coloured pins to represent non-bonding electron pairs (electron pairs that are not used for bonding) and compare electron-domain geometry with molecular shape.
Activity 2: Introducing Molymods
Molymod Models
Explore molecular geometry further by building methane, ammonia, and water with molymods.
Go to the Molview website on your device (www.molview.com) and examine the structures of the molecules that you are building with MolyMods. Use the Model tab to change the view from "ball and stick" to "Van der Waals spheres". Which do you think is closer to the "real" shape of a molecule.
Explore a few more molecules, such as ethane, butane, benzene and ethanol.
- Compare your ball-and-stick models with space-filling representations in MolView.
- Discuss: are molymods a good representation of molecules? What do they show well, and what do they miss?
Drawing Molecules
We also need a way to represent molecules on paper. Chemists use Lewis structures (dot-cross diagrams) to show bonding pairs and lone pairs of electrons. These diagrams connect the visual models with the abstract electron count.
Examples
CH4 (Methane)
four single bonds, tetrahedral shape.
NH3 (Ammonia)
three single bonds and one lone pair, trigonal pyramidal.
H2O (Water)
two single bonds and two lone pairs, bent shape.
Summary
- Carbon forms four covalent bonds; electron pairs arrange to minimise repulsion.
- Electron-domain geometry (pairs) is not always the same as the molecular shape (atoms).
- Models (pins, molymods, MolView) and Lewis structures connect counting electrons to 3D shape.
Check your understanding
- Explain why carbon forms four covalent bonds (tetravalency) in terms of valence electrons.
- What is the difference between electron-domain geometry and molecular shape? Give an example.
- State the typical bond angle for a tetrahedral arrangement and outline (in words) why it arises.
C4.2 - Allotropes of Carbon
Many elements exist in more than one physical form called allotropes. Changes in stucture and bonding between atoms explain dramatic differences in properties such as hardness, conductivity, and stability.
Carbon allotropes are remarkable in their differences even though they all contain only carbon atoms.
Carbon allotropes
Model & Compare
- Diamond – 3D network (each C is tetrahedral); very hard, electrical insulator.
- Graphite – 2D layers (sp2 trigonal planar) with delocalised electrons; soft, conducts along layers.
- Graphene – single graphite layer; very strong, excellent conductor.
- Fullerenes / nanotubes – curved cages/tubes with unusual mechanical/electronic properties.
Using the Molymods, build small lattice fragments (diamond tetrahedra; graphite hexagonal sheet). Sketch graphene, fullerene (C60), and a nanotube.
Graphene
Graphene is considered a new substance even though its been around for many years. When a pencil makes a mark on a piece of paper the black line consists of layers of graphite, called graphene.
Recently researchers found that these graphene layers have remarkable properties and if large sheets of graphene could be manufactured then many technological innovations would become possible.
Allotropy across other elements
Compare & Explain
Many elements have allotropes, the stability of which is dependent on the ambient pressure and temperature.
- Oxygen: O2 (stable) vs O3 ozone .
- Phosphorus has three common allotropes, white P (P4 tetrahedra; strained, reactive) → red P (polymeric, more stable) → black P (layered, most stable).
- Tin has two common allotropes, β-Sn (white, metallic, stable above ~13 °C) ⇄ α-Sn (grey, brittle, “tin pest”, stable below ~13 °C).
What does the term "stability" refer to in this context?
This is a fundamental concept in chemistry.
Case Study: Napoleon’s Buttons (Myth-busting)
The story claims tin buttons on uniforms crumbled in the 1812 Russian winter due to tin pest (β-Sn → α-Sn).
Evaluate the plausibility - fact check:
- Did soldiers uniform buttons actually contain tin?
- Were the temperatures low enough when Napoleon attacked Moscow?
- What alternative explanations exist?
Summary
Allotropy shows how the same element can display radically different behaviours when its atoms connect in different ways.
Understanding structure helps to explain properties and stability trends across elements.
Check your understanding
- Why does graphite conduct electricity while diamond does not?
- Why is the formation of ozone in the upper atmosphere essential to life on our planet?
- Why does tin undergo the α ⇄ β transition around 13 °C, and what consequence does this have in cold climates?
C4.3 Organic Nomenclature Basics (Part 1)
In this lesson you will learn the core language of organic chemistry: how to name simple molecules using roots and functional group suffixes. We focus on straight-chain compounds (C1–C6) before moving to branching and locants.
Roots and homologous series
Build the backbone
- Using molymods, build methane → hexane (C1–C6). Say the names out loud as you build.
- Record the general formula for the alkane homologous series.
- Discuss: how does adding one CH2 unit change properties (mass, b.p.)?
Functional group suffixes
Spot the group
- Add a double bond to make an alkene (–ene). Add a triple bond for an alkyne (–yne).
- Swap an H for –OH to make an alcohol (–ol). Swap an H for –Cl / –Br to make haloalkanes (chloro–, bromo–).
- Extend the chain and add –COOH to make a carboxylic acid (–oic acid).
For each build, write the name and draw a condensed structural formula.
Examples
Ethane → Ethene → Ethyne; Propane → 1-propanol; Butane → Chlorobutane; Ethanoic acid.
Summary
You can now name straight-chain C1–C6 molecules and recognise common functional groups. This vocabulary prepares you for branching, locants, and isomerism next lesson.
Check your understanding
- Name these: CH3–CH2–CH3; CH2=CH–CH3; CH≡C–CH3.
- Which suffix would you use for an alcohol? For a carboxylic acid?
- Give the general formula for alkanes and explain what “homologous series” means.
C4.4 Organic Nomenclature Basics (Part 2): Substitution, Locants & Branching
This lesson adds precision to your naming: numbering the chain (locants), mono/di-substitution, and simple branching. You’ll practise rapidly with a Molymod Challenge.
Locants and substitution
Number it right
- Choose the longest continuous carbon chain as the parent (e.g. butane, pentane).
- Number the chain to give the lowest set of locants to double/triple bonds and substituents.
- Apply mono-/di- prefixes (e.g. 1-chloropropane vs 1,2-dichloroethane).
Write names for teacher-provided structures, then swap: build each other’s named molecules to check accuracy.
Branching (alkyl substituents)
Branching is when the carbon chain forks into two different chains. A branch may have as few as one carbon atom.
The longest unbroken chain provides the root of the name of the compound.

Add side chains
- Introduce methyl– and ethyl– substituents. Example: 2-methylpropane, 3-methylpentane.
- Alphabetise different substituents; use di-/tri- for repeats (e.g. 2,3-dimethylbutane).
- Redraw neatly as condensed structural formulae after checking with molymods.
Examples
1-chloropropane; 2-chloropropane; 1,2-dichloroethane; 2-methylpropane; 2,3-dimethylbutane.
Let's go over nomenclature basics again
Molymod Challenge
Name ↔ Build Race
- Round A (Name → Build): Teacher calls “2-methylbutane”, “1-bromopropane”, “2-butanol”. Teams build fast, then hold up.
- Round B (Build → Name): Teacher shows a model; teams write the IUPAC name with correct locants.
- Round C (Isomer Sprint): “Make a different isomer of C5H12.” Bonus for neat, unambiguous condensed formulas.
Summary
You can now number chains, position functional groups, and name simple branched molecules accurately. Next, you’ll measure boiling points and connect structure to intermolecular forces.
Check your understanding
- Give correct names (with locants): CH3–CH(Cl)–CH3 and CH3–CH2–CH2Cl.
- Name a different isomer of C5H12 than pentane, and draw its condensed formula.
- Explain what “lowest set of locants” means and apply it to 2,3-dimethylbutane.
Activity - The Nomenclature Olympics
Each student starts with:
- 8 carbon atoms (black - four holes)
- 18 hydrogen atoms (white - one hole)
- 4 oxygen atoms (red - two holes)
- 4 chlorine atoms (green - one hole)
- 18 short (single) bonds
- 4 long (double) bonds.
Use the link below to open up the application. Enter the agreed time and click "next molecule" to start the race.
Students will go head-to-head to build the molecules displayed on the screen.
To turn off the alarm, click on the timer.
Winners go through to the next round.
The program will take about 30 seconds to load - please be patient!
C4.5 - Unsaturation
Although the term suggests something to do with water, it only refers to compounds containing either double or triple carbon - cartbon bonds.
They are said to be "unsaturated" in that they are able to bond more hydrogen (or other atoms) by opening the double or triple bonds.
We have seen in the section on nomenclature that carbon carbon double bonds give molecules the suffix "-ene", and triple bonds "-yne".
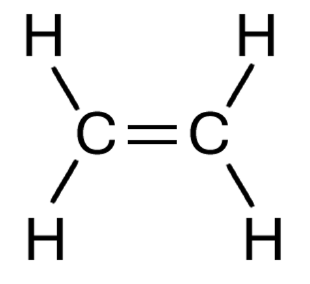
The displayed structure of ethene
Physical properties
The alkenes have very similar physical properties to alkanes. The smaller members of the homologous series are volatile compounds that are gases at room temperature and after C5H10, pentene they are liquids. The alkenes do not dissolve in water to any great extent, but dissolve in non-polar solvents.
Chemical properties
Alkenes are more reactive than alkanes due to the presence of the double bond. This can be used to differentiate the two families of compounds.
Activity - Testing for unsaturation
Pour a few cm3 of bromine water into a clean test tube.
Add a few drops of hex-1-ene. Place a bung in the test tube and shake. Observe any change
To a second clean test tube with bromine water, add a few drops of hexane. Place a bung in the test tube and shake. Observe any change
How do the hexane and hex-1-ene behave with the bromine water?
Repeat the tests above using potassium manganate(VII) instead of bromine water
How do the hexane and hexene behave with potassium manganate(VII)?
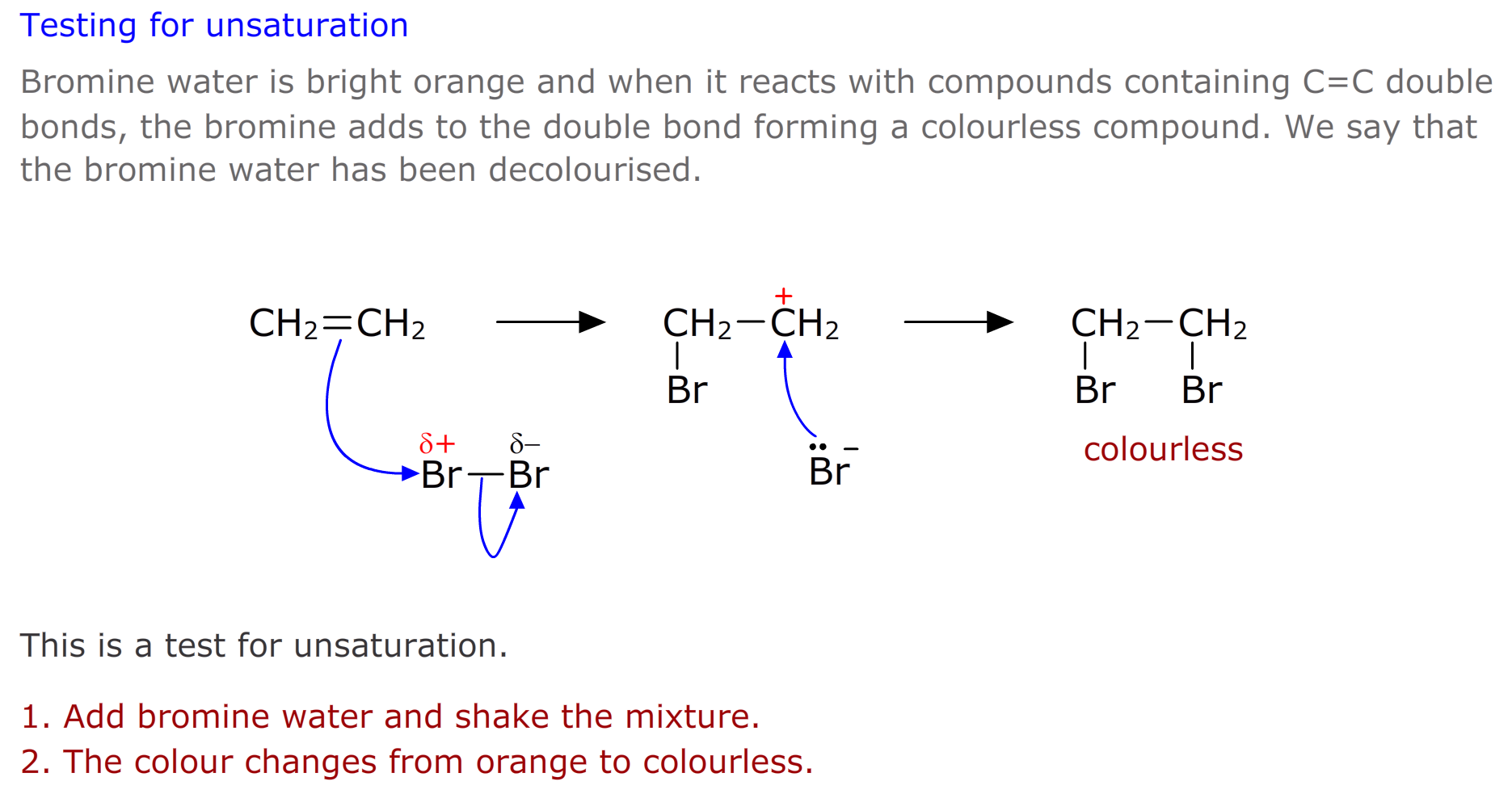
Tesing for unsaturation
Activity - Making ethyne
Place a small spatula of calcium carbide into a clean boiling tube (large test tube).
Using a water bottle add a few drops of water.
Rest a rubber bung on top of the test tube (do NOT push it into the tube)
Test the gas using a lighted splint - CARE
Add a few cm3 of water to the remaining calcium carbide. Allow the reaction to subside and filter the liquid into a clean test tube.
Test the pH of the filtrate by placing one drop onto a piece of indicator paper.
Carefully use a clean straw to breathe exhaled air gently (no splashing) through the filtrate. Observe.
Now test yourself
Click on the button below to access the self-tests for MYP9 and MYP10.